Step-by-step explanation:
Total volume of the solution is as follows.
19 ml + 19 ml = 38 ml
As, density is the mass divided by volume.
Mathematically, Density =
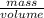
Now, calculate the mass of solution as follows.
mass of solution = Density × volume
= 1.00 g/ml × 38 ml
= 38 g
Specific heat of the solution is 4.184 J/g°C.
Relation between heat energy, mass, specific heat and temperature change temperature as follows.
Q =
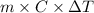
=
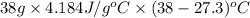
= 1701.21 J
Now, milimoles = molarity × volume
=
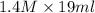
= 26.6 mmol
Enthalpy of neutralization is as follows.
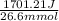
= 63.95 J/mmol
or, = 64 J/mmol
Thus, we can conclude that the enthalpy of neutralization in Joules/mmol for given solution is 64 J/mmol.