Answer: 0.069 mmol/L
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
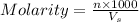
where,
n= moles of solute =
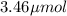 =
=
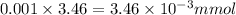
1micro (µ) = 0.001 milli (m)
 = volume of solution in ml= 50 ml
= volume of solution in ml= 50 ml
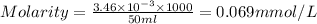
Thus concentration in mmol/L of the chemist's potassium dichromate solution is 0.069.