Answer:
3 Fe₂O₃(s) + H₂(g) ⇒ 2 Fe₃O₄(s) + H₂O(g) ΔH° = -6.00 kJ
Step-by-step explanation:
Let's consider the following balanced equation.
3 Fe₂O₃(s) + H₂(g) ⇒ 2 Fe₃O₄(s) + H₂O(g)
When 1 mole of Fe₂O₃(s) reacts, 2.00 kJ of energy are evolved. Energy is an extensive property. In the balanced equation there are 3 moles of Fe₂O₃(s), so the evolved energy is:
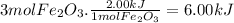
By convention, when energy is evolved it takes the negative sign. At constant pressure, the thermochemical equation is:
3 Fe₂O₃(s) + H₂(g) ⇒ 2 Fe₃O₄(s) + H₂O(g) ΔH° = -6.00 kJ
where
ΔH° is the standard enthalpy of reaction (heat released at constant pressure)