Step-by-step explanation:
Given that,
(a) Speed,

Mass of the electron,
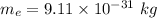
Mass of the proton,
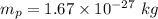
The wavelength of the electron is given by :
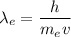
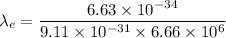
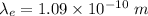
The wavelength of the proton is given by :
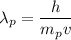
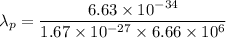
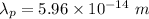
(b) Kinetic energy,
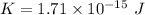
The relation between the kinetic energy and the wavelength is given by :
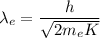
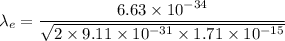
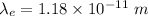
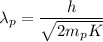
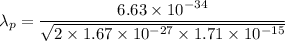
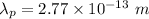
Hence, this is the required solution.