Answer:
77.83783 °C
Step-by-step explanation:
V = Volume of room = 36 m²
c = Heat capacity = 1000 J/kg C
P = Power = 120 J/s = 120 W
t = Time = 8 hours
 = Density of air = 1.225 kg/m³
= Density of air = 1.225 kg/m³
 = Change in temperature
= Change in temperature
Density is given by
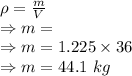
Heat is given by
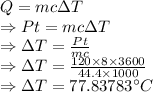
The temperature change you expected in this air 77.83783 °C