Answer:
1086.8 torr
Step-by-step explanation:
Using Boyle's law
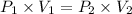
Given ,
V₁ = 0.671 L
V₂ = 583 mL = 0.583 L ( 1 mL = 0.001 L )
P₁ = 1.24 atm
P₂ = ?
Using above equation as:
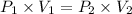
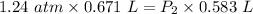
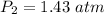
The conversion of P(atm) to P(torr) is shown below:
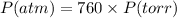
So,
Pressure = 1.43*760 torr = 1086.8 torr