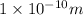Answer: The wavelength for X-rays with the given frequency is
Explanation:
To calculate the wavelength of light, we use the equation:
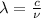
where,
 = wavelength of the light
= wavelength of the light
c = speed of light =
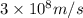
 = frequency of light =
= frequency of light =
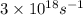
Putting the values in above equation, we get:
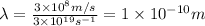
Hence, the wavelength for X-rays with the given frequency is