Answer: Option (B) is the correct answer.
Step-by-step explanation:
According to the given reaction equation, formula to calculate
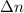 is as follows.
is as follows.
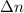 = coefficients of gaseous products - gaseous reactants
= coefficients of gaseous products - gaseous reactants
= 1 - 0
= 1
Also we know that,
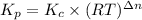
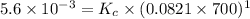
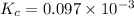
For the equation,
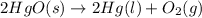
Activity of solid and liquid = 1
As,
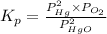
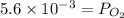
Hence,
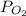 = 0.0056 atm
= 0.0056 atm
Thus, we can conclude that partial pressure of oxygen gas at equilibrium is 0.0056 atm.