Answer:
The correct answer is D) 7.081
Step-by-step explanation:
The water equilibrium is the following:
H₂O ⇄ H⁺ + OH⁻ Kw
Where Kw= [H⁺] x [OH⁻]
In pure water: [H⁺] = [OH⁻]= C
If we introduce C in the expression for Kw:
Kw= [H⁺] x [OH⁻]= C x C= C²
⇒ C=
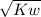 =
=
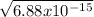 = 8.29 x 10⁻⁸
= 8.29 x 10⁻⁸
pH= -log [H⁺] = -log C = -log (8.29 x 10⁻⁸) = 7.081