Answer:
28.108 K.
Explanation:
Given: Pressure (P)= 1.2atm
Number of moles (n)= 2.6 moles
Volume (V)= 5.00-L
Now finding the temperature (T).
Formula; T=
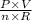
R is a constant factor which makes other factors work together.
There is a numerical value for R which we use is

∴ Temperature (T)=
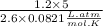
⇒ Temperature (T)=
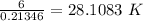
∴ Temperature is 28.108 K