Step-by-step explanation:
To convert moles to particles or grams to particles, let us have a firm understanding of what a mole is.
A mole is the unit of measuring quantity of particles.
It is the amount of substance that contains the Avogadro's number of particles.
The particle can be atoms, molecules, formula units, electrons, protons, neutrons, etc.
So, to convert from moles to particles;
1 mole of a substance contains 6.02 x 10²³ particles
To convert from grams to particles;
First convert to moles;
number of moles =
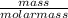
So, 1 mole of a substance contains 6.02 x 10²³ particles