Answer: The mass of hydrogen sulfide that can be dissolved is 2.86 grams.
Step-by-step explanation:
Henry's law states that the amount of gas dissolved or molar solubility of gas is directly proportional to the partial pressure of the gas.
To calculate the molar solubility, we use the equation given by Henry's law, which is:
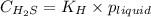
where,
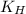 = Henry's constant =
= Henry's constant =
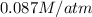
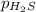 = partial pressure of hydrogen sulfide gas = 2.42 atm
= partial pressure of hydrogen sulfide gas = 2.42 atm
Putting values in above equation, we get:
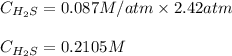
To calculate the mass of solute, we use the equation used to calculate the molarity of solution:
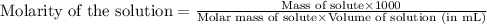
We are given:
Molarity of solution = 0.2105 M
Molar mass of hydrogen sulfide = 34 g/mol
Volume of solution = 400.0 mL
Putting values in above equation, we get:
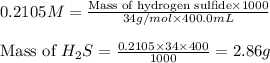
Hence, the mass of hydrogen sulfide that can be dissolved is 2.86 grams.