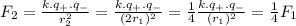Answer:
one-quarter as strong
Step-by-step explanation:
Coulomb's law gives the mathematical expression to calculate the electrical force (F) between the charge of the nucleus (q₊) and the charge of an electron (q₋) separated by a distance (r).
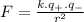
where,
k is the Coulomb's constant
The force between the nucleus and an electron in the level 1 is:
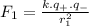
Considering the distance to an electron from n = 2 is twice as great as the distance to an electron from n = 1, the force between the nucleus and an electron in the level 2 is: