Answer : The correct option is, two reactants and two products.
Explanation :
Double replacement reaction : It is a type of chemical reaction in which a positive cation and a negative anion of two reactants exchange their places to form two new products.
It is represented as,
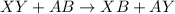
(X and A are the cations, Y and B are the anions)
For example : Barium chloride react with magnesium sulfate to give barium sulfate and magnesium chloride as a product.
The balanced chemical reaction will be:
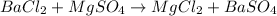
From this we conclude that, there are two reactants and two products are present in a double-replacement reaction.
Hence, the correct option is, two reactants and two products.