Answer:
c)The gases have the same average kinetic energy.
Step-by-step explanation:
As we know that the kinetic energy of gas is given as
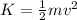
here we know that
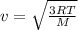
so we have
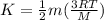
now we have
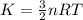
now mean kinetic energy per molecule is given as
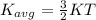
so this is independent of the mass of the gas
so average kinetic energy will remain same for both the gas molecules