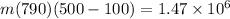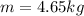Answer:
m = 4.65 kg
Step-by-step explanation:
As we know that the mass of the water that evaporated out is given as
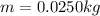
so the energy released in form of vapor is given as
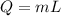
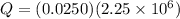
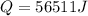
now the heat required by remaining water to bring it from 15 degree to 100 degree
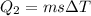
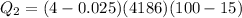
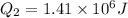
total heat required for above conversion
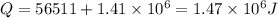
now by heat energy balance
heat given by granite = heat absorbed by water