Answer: The mole fraction of hydrogen gas at 20°C is 0.975
Step-by-step explanation:
We are given:
Vapor pressure of water vapor at 20°C = 17.5 torr
Total pressure at 20°C = 700.0 torr
Vapor pressure of hydrogen gas at 20°C = (700.0 - 17.5) torr = 682.5 torr
To calculate the mole fraction of hydrogen gas at 20°C, we use the equation given by Raoult's law, which is:
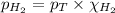
where,
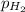 = partial pressure of hydrogen gas = 682.5 torr
= partial pressure of hydrogen gas = 682.5 torr
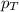 = total pressure = 700.0 torr
= total pressure = 700.0 torr
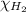 = mole fraction of hydrogen gas = ?
= mole fraction of hydrogen gas = ?
Putting values in above equation, we get:
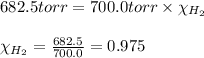
Hence, the mole fraction of hydrogen gas at 20°C is 0.975