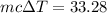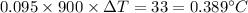Answer:
Increase in temperature will be
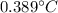
Step-by-step explanation:
We have given mass of the aluminium m = 0.095 kg
Height h = 55 m
Specific heat of aluminium c = 900 J/kg°C
We know that potential energy is given as
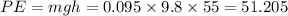
Now 65 % of potential energy
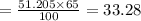
Now this energy is used to increase the temperature
So