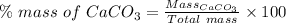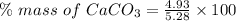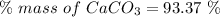Answer:
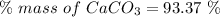
Step-by-step explanation:
Given that:
Pressure = 791 mmHg
Temperature = 20.0°C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T = (20 + 273.15) K = 293.15 K
T = 293.15 K
Volume = 100 L
Using ideal gas equation as:
PV=nRT
where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 62.3637 L.mmHg/K.mol
Applying the equation as:
791 mmHg × 1.14 L = n × 62.3637 L.mmHg/K.mol × 293.15 K
⇒n of
 produced = 0.0493 moles
produced = 0.0493 moles
According to the reaction:-
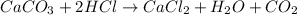
1 mole of carbon dioxide is produced 1 mole of calcium carbonate reacts
0.0493 mole of carbon dioxide is produced 0.0493 mole of calcium carbonate reacts
Moles of calcium carbonate reacted = 0.0493 moles
Molar mass of
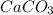 = 100.0869 g/mol
= 100.0869 g/mol
The formula for the calculation of moles is shown below:

Thus,
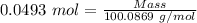
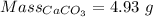
Impure sample mass = 5.28 g
Percent mass is percentage by the mass of the compound present in the sample.