Answer: a ) NaCl
b) No compound.
Step-by-step explanation:
Atomic number of A is 11 , thus it is sodium. It has electronic configuration of 2,8,1 and thus has 1 valence electron and can form
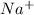 .
.
Atomic number of B is 18 , thus it is argon. It has electronic configuration of 2,8,8 and thus it is an inert gas.
Atomic number of C is 1 , thus it is hydrogen. It has electronic configuration of 1. It can only share electrons.
Atomic number of D is 17 , thus it is chlorine. It has electronic configuration of 2,8,7 and thus has 7 valence electron and can form
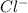 .
.
a) A and D :
Here Sodium is having an oxidation state of +1 called as
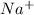 cation and anion is
cation and anion is
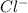 . Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
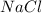
b) B and C
As B is an inert gas , it wont combine with C.