Step-by-step explanation:
The given data is as follows.
Molar mass of ammonium nitrate = 80.0 g/mol
So, we will calculate the number of moles of ammonium nitrate as follows.
No. of moles =
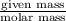
=
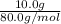
= 0.125 mol
Heat released due to solution of ammonium nitrate =
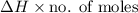
=
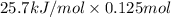
= 3.2125 KJ
= 3212.5 J (as 1 kJ = 1000 J)
Therefore, calculate the total mass of solution as follows.
mass of solution(m) = (10.0 + 100.0 ) g
= 110.0 g
Hence, heat released will be calculated as follows.
Q =
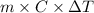
3212.5 J =
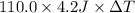
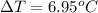
Thus, we can conclude that the change in temperature of the solution is
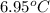 .
.