Answer:
Silicon
Step-by-step explanation:
The formula for the calculation of the average atomic mass is:
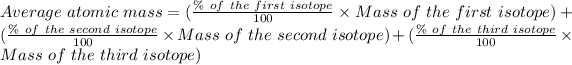
Given that:
For first isotope, X-28 :
% = 92.23 %
Mass = 27.977 amu
For second isotope, X-29:
% = 4.67 %
Mass = 28.976 amu
For third isotope, X-30:
% = 3.10 %
Mass = 29.974 amu
Thus,
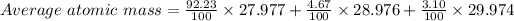
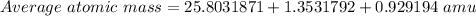
Average atomic mass = 28.0855603 amu ≅ 28.086 amu
This mass corresponds to Silicon, ( Z = 14).