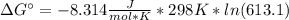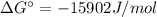Answer:
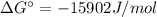
Step-by-step explanation:
In this problem we only have information of the equilibrium, so we need to find a expression of the free energy in function of the constant of equilireium (Keq):
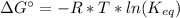
Being Keq:
![K_(eq)=([fructose][Pi])/([Fructose-1-P])](https://img.qammunity.org/2020/formulas/chemistry/college/3wmj9641si5dd9xofbgo72dtf8nwbw8cq1.png)
Initial conditions:
![[Fructose-1-P]=0.2M](https://img.qammunity.org/2020/formulas/chemistry/college/kmpms6pkggecdxq0i28jm3epoksoy5y2bg.png)
![[Fructose]=0M](https://img.qammunity.org/2020/formulas/chemistry/college/hr5ds41gdkvp2bjzaug62mns4ah6918zq7.png)
![[Pi]=0M](https://img.qammunity.org/2020/formulas/chemistry/college/knnx7bk1baxgstny5vwgmnbx87vdzh8nj7.png)
Equilibrium conditions:
![[Fructose-1-P]=6.52*10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/college/9s7vch3iw5ua71t6wqd7li1k41kke0i51g.png)
![[Fructose]=0.2M-6.52*10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/college/wq0h3qe9iebdqycuk6bnbayb67b7zqmj5c.png)
![[Pi]=0.2M-6.52*10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/college/x9pzo5xh9m5ex8g6zn3uh59lpuef0pb20m.png)
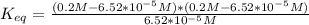
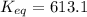
Free-energy for T=298K (standard):