Step-by-step explanation:
The given data is as follows.
50 ml of
 , 50 ml of
, 50 ml of
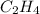
And, it is known that at STP 1 mole of a gas occupies 22.4 L. Hence, moles present in 50 ml of gas are as follows.
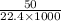 (As 1 L = 1000 ml)
(As 1 L = 1000 ml)
=
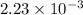 moles
moles
So, according to the given equation
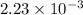 moles of
moles of
 reacts with
reacts with
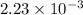 moles of
moles of
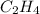 .
.
Hence, moles of
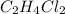 is equal to the moles of
is equal to the moles of
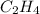 and
and
 .
.
Therefore, moles of
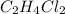 =
=
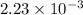 moles
moles
1 mole of
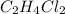 = 22.4 L
= 22.4 L
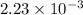 moles =
moles =

= 50 ml of product
Thus, we can conclude that 50 ml of products if pressure and temperature are kept constant.