Step-by-step explanation:
As it is given that both the given containers are at same temperature and pressure, therefore they have the same density.
So, mass of
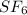 in container- 1 is as follows.
in container- 1 is as follows.
5.35 mol x molar mass of
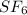
= 7.61 mol x 146.06 g/mol
= 1111.52 g
Therefore, density of
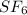 will be calculated as follows.
will be calculated as follows.
Density =

density =
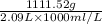
= 0.532 g/mL
Now, mass of
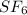 in container- 2 is calculated as follows.
in container- 2 is calculated as follows.
4.46 L x 1000 mL/L x 0.532 g/mL
= 2372.72 g
Hence, calculate the moles of moles
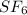 present in container 2 as follows.
present in container 2 as follows.
No. of moles =

=
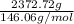
= 16.24 mol
Since, 1 mol
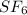 contains 6 moles F atoms .
contains 6 moles F atoms .
So, 16.24 mol
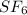 contains following number of atoms.
contains following number of atoms.
=
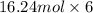
= 97.46 mol
Thus, we can conclude that moles of F atoms in container 2 are 97.46 mol.