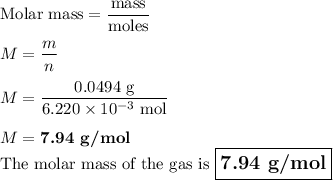
Answer:
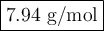
Step-by-step explanation:
We can use the Ideal Gas Law to solve this problem
pV = nRT
Data:
p = 0.998 atm
V = 0.153 L
T = 26 °C
m = 0.0494 g
1. Convert temperature to kelvins
T = (26 + 273.15) K = 299.15 K
2. Calculate the number of moles
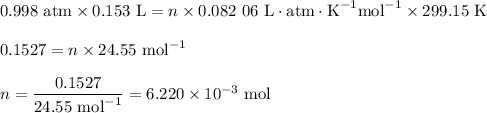
3. Calculate the molar mass