Answer:
A. Alpha decay followed by emission
Step-by-step explanation:
The given nuclide is thorium-232
The product formed in the decay of thorium-232 is actinium -228
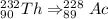
the difference between the atomic number is 1 (90-89)
the difference between the mass number is 1 (232-228)
A decrease in mass number by 4 and atomic number by 2 indicates an alpha decay.
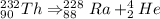
now to get ^{228}_{89}Ac from ^{228}_{88}Ra, Radium undergoes beta emission to increase the atomic number by 1 and no change in the mass number.
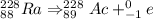
Thus alpha decay followed by beta emission gives Ac-228
hence option A is correct