Answer:
The concentration of the H₃PO₄ solution is 0,245M
Step-by-step explanation:
The first titration is:
KHP + NaOH → KP⁻ + Na⁺ + H₂O
0,495g of KHP are:
0,495g×
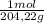 = 2,42x10⁻³ moles of KHP
= 2,42x10⁻³ moles of KHP
As 1 mole of KHP reacts with 1 mole of NaOH, moles of NaOH are 2,42x10⁻³ moles.
As volume required was 28,56mL, the concentration of the NaOH solution is:
2,42x10⁻³ moles / 0,02856L = 0,0849M
The titration of the phosporic acid with NaOH occurs as follows:
H₃PO₄ + NaOH → H₂PO₄⁻ + Na⁺ + H₂O
If were required 29,88mL of NaOH, the moles of NaOH spent were:
0,0849M×0,02988L = 2,54x10⁻³ moles of NaOH that are the same than H₃PO₄ moles.
As the volume of the solution of H₃PO₄ was 10,33mL, the concentration of the H₃PO₄ solution is:
2,54x10⁻³ moles of H₃PO₄ / 0,01033L = 0,245M
I hope it helps!