Answer: Valency of Al in
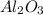 is +3.
is +3.
Step-by-step explanation:
The capacity of an element to gain or lose electrons is called valency of the atom or element.
The valency of oxygen is -2 is the compound
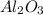 and let the valency of Al is x over here.
and let the valency of Al is x over here.
Therefore, valency of x will be calculated as follows.
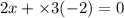 (as the charge on compound is 0)
(as the charge on compound is 0)
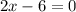
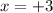
Therefore, valency of Al in
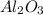 is +3.
is +3.