Answer:
Step-by-step explanation:
In one Ca(OH)₂ molecule, there are 5 atoms (1 Ca, 2 O and 2 H). This means that in 1.005x10²⁴ Ca(OH)₂ molecules, there are (5 * 1.005x10²⁴) 5.025x10²⁴ atoms.
To convert molecules into moles, we use Avogadro's number:
- 1.005x10²⁴ molecules *
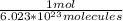 = 1.6686 moles
= 1.6686 moles
Finally, we convert moles into grams using the molar mass of Ca(OH)₂:
- 1.6686 moles * 74.093 g/mol = 123.63 g