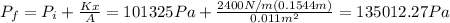Answer:
a) The correct solution would be x=0.1544m.
b)
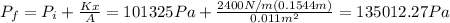
Step-by-step explanation:
Initial states
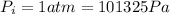 represent the initial pressure
represent the initial pressure
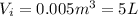 represent the initial volume
represent the initial volume
N represent the number of moles
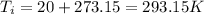 represent the initial temperature
represent the initial temperature
Final states
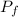 represent the final pressure
represent the final pressure
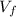 represent the final volume
represent the final volume
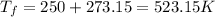
Part a
From the initial states and with the law of ideal gases we can find the moles like this
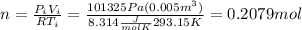
In order to find the final pressure we need to take in count the atmospheric pressure and the pressure related to the force that the springs applies to the piston, like this:
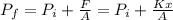
Where x is the distance that the piston is displaced upward, since increase the temperature and the gas inside tends to expand.
And we can find the final volume on this way
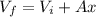
For the final state we have the following equation:
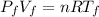
And if we replace
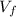 we got:
we got:
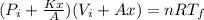
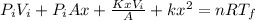
And x can be solved with a quadratic equation:
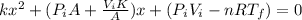
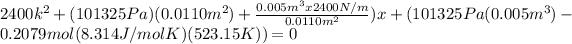
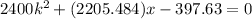
And solving for x we got x=0.1544 or x=-1.07331 m. The correct solution would be x=0.1544m.
Part b
And the final pressure would be given by: