Answer:
Final temperature T is 26.197 degrees celcius.
Step-by-step explanation:
As the system is in thermal equilibrium, the heat lost by aluminium is the heat gained by water.
specific heat capacity of aluminium = 0.9 J/g per degree celcius
specific heat capacity of water = 4.186 J/g per degree celcius
Let the final common temperature attained be "T".
Heat lost by aluminium =

Heat gained by water =
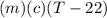
by law of conservation of energy,
Heat lost by hot body = Heat gained by cold body
 =
=
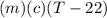
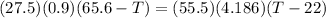
calculating the value of T comes out as 26.197 degrees celcius.