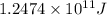Answer: The energy released for the the given amount of hydrogen -1 atom is
Step-by-step explanation:
First we have to calculate the mass defect
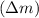 .
.
The given equation follows:
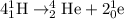
To calculate the mass defect, we use the equation:
Mass defect = Sum of mass of product - Sum of mass of reactant
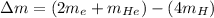
We know that:
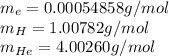
Putting values in above equation, we get:
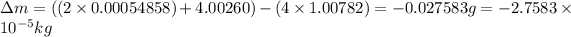
(Conversion factor: 1 kg = 1000 g )
To calculate the energy released, we use Einstein equation, which is:

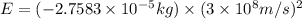
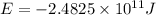
The energy released for 4 moles of hydrogen atom is
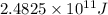
To calculate the number of moles, we use the equation:

Given mass of hydrogen atom = 2.01 g
Molar mass of hydrogen atom = 1 g/mol
Putting values in above equation, we get:
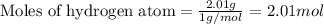
We need to calculate the energy released for the fusion of given amount of hydrogen atom. By applying unitary method, we get:
As, 4 moles of hydrogen atom releases energy of =
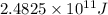
Then, 2.01 moles of hydrogen atom will release energy of =
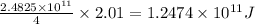
Hence, the energy released for the the given amount of hydrogen -1 atom is