Answer:
The % of displaced volume of nitrogen is 29.06%.
Step-by-step explanation:
Volume of nitrogen = 1.2 L = 1200 mL
Density of nitrogen = 0.807 g/ml
Mass of nitrogen =
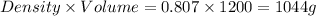
Molar mass of nitrogen = 28 g/mol
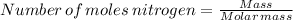
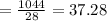
The ideal gas equation is as follows
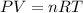
Rearrange the equation is as follows.
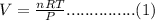
n= Number of moles = 37.28
R = Gas constant = 0.0820
T = Temperature = 22+ 273 = 295
P = Pressure = 1.2 atm
Substitute the all values in equation (1)
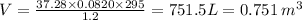
 of nitrogen will displace same amount of air.
of nitrogen will displace same amount of air.
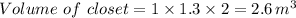

Therefore, The % of displaced volume of nitrogen is 29.06%.