Answer:
2.12 grams of primary-standard-grade sodium carbonate are necessary to prepare 800.0 mL of this solution.
Step-by-step explanation:
Molarity of sodium ions =
![[Na^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ra4gvqtntqnsbvg9qqnkx7i7d3czf4uh10.png) = 0.0500 M
= 0.0500 M
Moles of sodium ions = n
Volume of the solution = V = 800.0 mL = 0.800 L
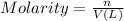
![[Na^+]=(n)/(V)](https://img.qammunity.org/2020/formulas/chemistry/high-school/1httjjivqqcuenb5hxxmyi6w1r0d22qomn.png)
![n=[Na^+]* V=0.0500 M* 0.800 L=0.04 mol](https://img.qammunity.org/2020/formulas/chemistry/high-school/uqe3ziqi0ciml5h671xyhefgw6ekccflhe.png)

1 mole sodium carbonates gives 2 moles of sodium ion and 1 mole of carbonate ions.
Then 0.04 moles of sodium ions will be obtained from:
 of sodium m carbonation.
of sodium m carbonation.
Mass of 0.02 moles of sodium carbonate = 0.02 mol × 106 g/mol= 2.12 g
2.12 grams of primary-standard-grade sodium carbonate are necessary to prepare 800.0 mL of this solution.