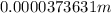Answer:
The mean free path is
Step-by-step explanation:
The formula for mean free path is :
λ =
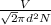
where,
λ - is the mean free path distance
V - volume of the gas
d - the diameter of the molecule
N - number of molecules.
now ,
density
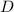 =
=
 =
=
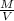 ;
;
mass of the gas = (number of molecules)
 (mass of one molecule) ;
(mass of one molecule) ;
as it's atomic hydrogen
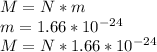
∴
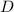 =
=
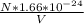
∴
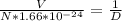
⇒ λ =
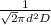
⇒ λ =
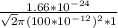
⇒ λ =