Answer:
q= 110.5 ke
Step-by-step explanation:
Dipole moment is the product of the separation of the ends of a dipole and the magnitude of the charges.
μ = q * d
μ= Dipole moment (1.93 D)
q= partial charge on each pole
d= separation between the poles(109 pm).
e= electronic charge ( 1.60217662 × 10⁻¹⁹ coulombs)
So,
q=
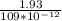 coulombs
coulombs
q =
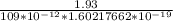 e
e
q = 1.105 * 10⁵ e
q= 110.5 ke