Answer:
Osmotic pressure(π) = 0.661 Torr.
Depression in freezing point = 6.64 * 10⁻⁵ °C.
Step-by-step explanation:
To calculate depression in freezing point and osmotic pressure, let's start by calculating Molarity of the solution.
Also, protein undergoes no dissociation or association when in solution.
Molarity =
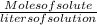
Molarity =
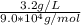
Molarity= 3.56 * 10⁻⁵ mol/L
Temperature = 25 +273 = 298 K
Osmotic pressure(π) = M R T
= (3.56 * 10⁻⁵ mol/L)* (0.08206 L atm/ mol K) * (298 K)
= 87.055 * 10⁻⁵ atm
But 1 atm= 760 Torr
So, Osmotic pressure(π) = (87.055 * 10⁻⁵ atm) * ( 760 torr/ 1atm)
= 0.661 Torr.
The depression in freezing point Δ
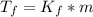
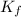 = molal freezing point depression constant of the solvent (1.86 °C/m for water).
= molal freezing point depression constant of the solvent (1.86 °C/m for water).
m= molality or molal concentration of the solution.
m= moles of solute in 1kg of solvent.
Density of solution =
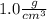
Mass of 1L(1000 cm³) of this solution is= density * volume of solution
= 1000g
Molarity means 3.56 * 10⁻⁵ mol of protein in 1L of solution
Mass of protein= 3.56 * 10⁻⁵ * 9.0 * 10⁴ = 3.2 g of protein
1000g of solution- 3.2 g of protein = 996.8 g of solvent
Molality =
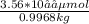
=3.57 * 10⁻⁵ m
depression in freezing point Δ
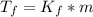
= 1.86 * 3.57 * 10⁻⁵ = 6.64 * 10⁻⁵ °C.