Answer:
The metal with a volume of
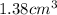 is zinc.
is zinc.
Step-by-step explanation:
Density is defined as mass of the substance present in the unit volume of the substance.
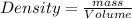
For aluminum:
Mass of aluminium metal , M = 4.60 g
Volume of the aluminium metal = V
Density of the aluminium metal = d =
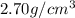
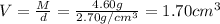
For Zinc :
Mass of zinc metal , M = 9.81 g
Volume of the zinc metal = V
Density of the zinc metal = d =
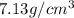
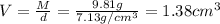
For chromium :
Mass of chromium metal , M= 6.24 g
Volume of the chromium metal = V
Density of the chromium metal = d =
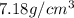
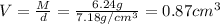
For nickel :
Mass of nickel metal , M= 3.17 g
Volume of the nickel metal = V
Density of the nickel metal = d =

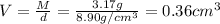
The metal with a volume of
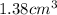 is zinc.
is zinc.