Answer:
336.8 kilo Joules is the change in internal energy of the system.
Step-by-step explanation:
The equation for first law of thermodynamics follows:
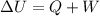
where,
Q = heat added to the system
ΔU = Change in internal energy
W = work done
We have :
Amount of heat given out by the system will be negatuive as heat relased by the system = Q
Q=

Work done on the system will positive as work is done on the system:
w =
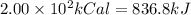
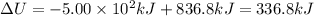
336.8 kilo Joules is the change in internal energy of the system.