Answer:
1.12M
Step-by-step explanation:
Given parameters:
Volume of solution = 2.5L
Mass of Calcium phosphate = 600g
Unknown:
Concentration = ?
Solution:
Concentration is the number of moles of solute in a particular solution.
Now, we find the number of moles of the calcium phosphate from the given mass;
Formula of calcium phosphate = Ca₃PO₄
molar mass = 3(40) + 31 + 4(16) = 215g/mol
Number of moles of Ca₃PO₄ =
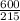 = 2.79moles
= 2.79moles
Now;
Concentration =
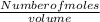
Concentration =
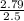 = 1.12M
= 1.12M