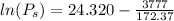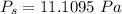Answer:
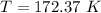
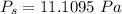
Step-by-step explanation:
Triple point is that temperature and pressure for a substance at which the chemical substance coexists in liquid, solid and gaseous state with mutual equilibrium.
- At this point the equilibrium vapor pressure of solid, liquid and gas phases are mutually equal.
Given expressions of vapor pressure:
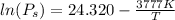 ........................................(1)
........................................(1)
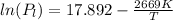 ..........................................(2)
..........................................(2)
Equating the vapor pressure of two phases of chlorine:
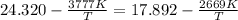
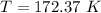
The vapor pressure at this temperature: