Answer: The standard enthalpy change for the given reaction is 868.05 kJ
Step-by-step explanation:
- Calculating the enthalpy of propane:
The chemical equation for the combustion of propane follows:
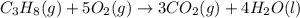
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(3* \Delta H^o_f_((CO_2(g))))+(4* \Delta H^o_f_((H_2O(g))))]-[(1* \Delta H^o_f_((C_3H_8(g))))+(5* \Delta H^o_f_((O_2(g))))]](https://img.qammunity.org/2020/formulas/chemistry/college/1m54j7bh1qx42vvk8uiweatn1jkr3is1z4.png)
We are given:
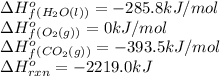
Putting values in above equation, we get:
![-2219.0=[(3* (-393.5))+(4* (-285.8))]-[(1* \Delta H^o_f_((C_3H_8(g))))+(5* (0))]\\\\\Delta H^o_f_((C_3H_8(g)))=-140.7kJ/mol](https://img.qammunity.org/2020/formulas/chemistry/college/mpblm078i49oeaoi3uc7kr3b3coscw5q0q.png)
The enthalpy of formation of
 is -140.7 kJ/mol
is -140.7 kJ/mol
- Calculating the enthalpy of propylene:
The chemical equation for the combustion of propane follows:
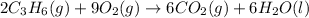
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(6* \Delta H^o_f_((CO_2(g))))+(6* \Delta H^o_f_((H_2O(g))))]-[(2* \Delta H^o_f_((C_3H_6(g))))+(9* \Delta H^o_f_((O_2(g))))]](https://img.qammunity.org/2020/formulas/chemistry/college/kkwz6jo01ztm2y4x3a4rhree10vd9krjad.png)
We are given:
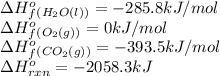
Putting values in above equation, we get:
![-2058.3=[(6* (-393.5))+(6* (-285.8))]-[(2* \Delta H^o_f_((C_3H_6(g))))+(9* (0))]\\\\\Delta H^o_f_((C_3H_6(g)))=-1008.75kJ/mol](https://img.qammunity.org/2020/formulas/chemistry/college/o127krr5op173vq5momdvwwp9pfw9ta2yi.png)
The enthalpy of formation of
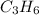 is -1008.75 kJ/mol
is -1008.75 kJ/mol
- Calculating the enthalpy change of the reaction:
The given chemical equation follows:
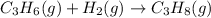
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(1* \Delta H^o_f_((C_3H_8(g))))]-[(1* \Delta H^o_f_((C_3H_6(g))))+(1* \Delta H^o_f_((H_2(g))))]](https://img.qammunity.org/2020/formulas/chemistry/college/tpy3c7oc8kslpvfz1aajwgvamd64sqa7l5.png)
We are given:
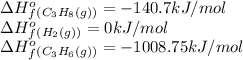
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(1* (-140.7))]-[(1* (-1008.75))+(1* (0))]\\\\\Delta H^o_(rxn)=868.05kJ](https://img.qammunity.org/2020/formulas/chemistry/college/2j03mxk538kgart6r9t9czyymibarplhrs.png)
Hence, the standard enthalpy change for the given reaction is 868.05 kJ