Answer:
1)50.007 grams of an isotope will left after 10 years.
1)25.007 grams of an isotope will left after 20 years.
3) 23 half-lives will occur in 40 years.
Step-by-step explanation:
Formula used :
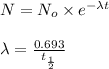
where,
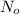 = initial mass of isotope
= initial mass of isotope
N = mass of the parent isotope left after the time, (t)
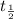 = half life of the isotope
= half life of the isotope
 = rate constant
= rate constant
We have:
![[N_o]=100 g](https://img.qammunity.org/2020/formulas/chemistry/high-school/eaj6gy0lrv6x2h1mjlpuyclhd5914raijq.png)
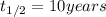
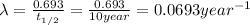
1) mass of isotope left after 10 years:
t = 10 years
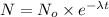
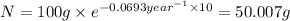
2) mass of isotope left after 20 years:
t = 20 years
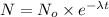
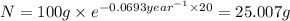
3) Half-lives will occur in 40 years
Mass of isotope left after 40 years:
t = 40 years
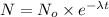
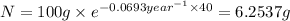
number of half lives = n
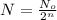
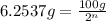
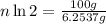
n = 23
23 half-lives will occur in 40 years.