Answer:
A)Mass of gallium plated out is 0.3440 grams
B) For 0.67 hours current of 5.79 A must to be applied to plate out 8.70 g of tin.
Step-by-step explanation:
To calculate the total charge, we use the equation:
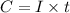
where,
C = Charge
I = Current in time t (seconds)
To calculate the moles of electrons, we use the equation:
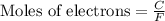
where,
F = Faraday's constant = 96500
A) The equation for the deposition of Ga(s) from Ga(III) solution follows:
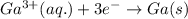
I = 0.790 A, t = 30.0 min = 1800 seconds
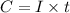
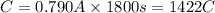
Moles of electron transferred:
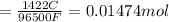
Now, to calculate the moles of gallium, we use the equation:
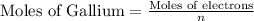
n = number of electrons transferred = 3
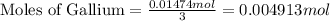
Mass of 0.004913 moles of gallium = 0.004913 mol × 70 g/mol=0.3440 g
B) The equation for the deposition of Sn(s) from Sn(II) solution follows:
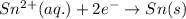
Moles of tin =
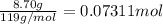
n = number of electrons transferred = 2
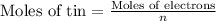
Moles of electron =

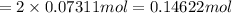
Charge transferred during time t :
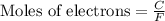
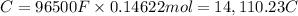
Current applied for t time = I = 5.79 A
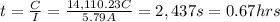
For 0.67 hours current of 5.79 A must to be applied to plate out 8.70 g of tin.