Answer:
102.807 kPa
Step-by-step explanation:
There are some assumptions to be made in the answer. The air inside the balloon acts as an ideal gas at a given temperature conditions.
Using the combined ideal gas equation.
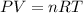
P= absolute pressure of air inside the balloon.
V= volume of air inside the balloon (6.23 L= 6.23 * 10⁻³ m³)
n= moles of gas(air). (0.250 mol)
R= Universal gas constant ( 8.314 J / mol·K)
T= Temperature in Kelvin
T= 35 + 273.15 = 308.15 K
So,
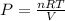
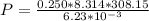
P= 102.807 * 10³ Pa
P= 102.807 kPa