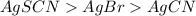Answer: The decreasing order of
 is
is
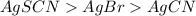
Step-by-step explanation:
The balanced equilibrium reaction for the ionization of silver bromide follows:
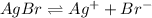
s s
The expression for solubility constant for this reaction will be:
![K_(sp)=[Ag^(+)][Br^-]](https://img.qammunity.org/2020/formulas/chemistry/college/ljl7gvndjeua79krr43izzsz5nhd7bsg8r.png)
We are given:
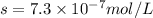
Putting values in above equation, we get:
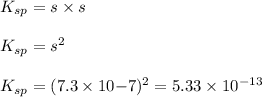
Solubility product of AgBr =
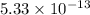
The balanced equilibrium reaction for the ionization of silver cyanide follows:
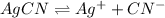
s s
The expression for solubility constant for this reaction will be:
![K_(sp)=[Ag^(+)][CN^-]](https://img.qammunity.org/2020/formulas/chemistry/college/2i9ae0m5cj4k05ex2l5obgw8pgs7zwladt.png)
We are given:
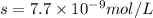
Putting values in above equation, we get:
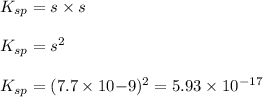
Solubility product of AgCN =
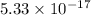
The balanced equilibrium reaction for the ionization of silver thiocyanate follows:
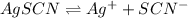
s s
The expression for solubility constant for this reaction will be:
![K_(sp)=[Ag^(+)][SCN^-]](https://img.qammunity.org/2020/formulas/chemistry/college/rv3liboy8sw27t7re98e08ogca22ssc3va.png)
We are given:
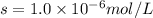
Putting values in above equation, we get:
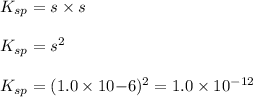
Solubility product of AgSCN =
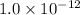
The decreasing order of
 follows:
follows: