Answer: -134 kJ
Step-by-step explanation:
The balanced chemical reaction is,
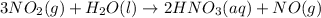
The expression for enthalpy change is,
![\Delta H=\sum [n* \Delta H_f(product)]-\sum [n* \Delta H_f(reactant)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/22ydrpaznpvv8tufv1zxgfrc1llt92w0u6.png)
![\Delta H=[(n_(HNO_3)* \Delta H_(HNO_3))+(n_(NO)* \Delta H_(NO))]-[(n_(H_2O)* \Delta H_(H_2O))+(n_(NO_2)* \Delta H_(NO_2))]](https://img.qammunity.org/2020/formulas/chemistry/college/dlyj3uvrmdj9fgfjsti14lxikt1x1iiahh.png)
where,
n = number of moles
Now put all the given values in this expression, we get
![\Delta H=[(2* -207)+(1* 90)]-[(1* -286)+(3* 32)]](https://img.qammunity.org/2020/formulas/chemistry/college/xxl2t8zykk2kd22a19k1wz4v5hthz3vq3t.png)

Therefore, the enthalpy change for this reaction is, -134 kJ