Answer: Option (A) is the correct answer.
Step-by-step explanation:
It is known that relation between pressure, density, height, and gravity is as follows.
P =
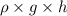
As it is given that pressure of both mercury and water column are equal. Therefore,
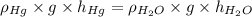
Cancelling the common terms in the formula. Now, putting the given values into the above formula as follows.
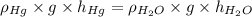
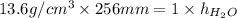
3481.6 mm =
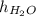
As 1 mm = 0.1 cm
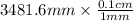
= 348.1 cm
or, = 348 cm (approx)
thus, we can conclude that height of the given column is 348 cm.