Answer: 656.6 nm.
Step-by-step explanation:
Using Rydberg's Equation for hydrogen atom:
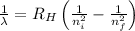
Where,
 = Wavelength of radiation
= Wavelength of radiation
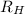 = Rydberg's Constant
= Rydberg's Constant
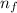 = Higher energy level = 3 (least energetic for visible series)
= Higher energy level = 3 (least energetic for visible series)
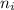 = Lower energy level = 2
= Lower energy level = 2
We have:
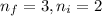
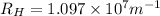
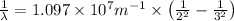
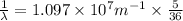
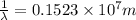
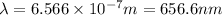
(
 )
)
The wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 2 to n = 3 is 656.6 nm.